Research projects
Our lab sits at the intersection of manufacturing engineering, bioengineering, materials science, and medicine—pioneering smart, personalized solutions for complex injuries through cutting-edge technologies like 3D printing, bioprinting, and extracellular vesicle-based therapies. We have several projects that specializes in 3D Printing/Bioprinting, biomaterials development, and regenerative medicine for musculoskeletal and bone defects and injuries. Of note, there are three are three primary projects that are currently under progress in the Sikder Lab, as mentioned in the following.
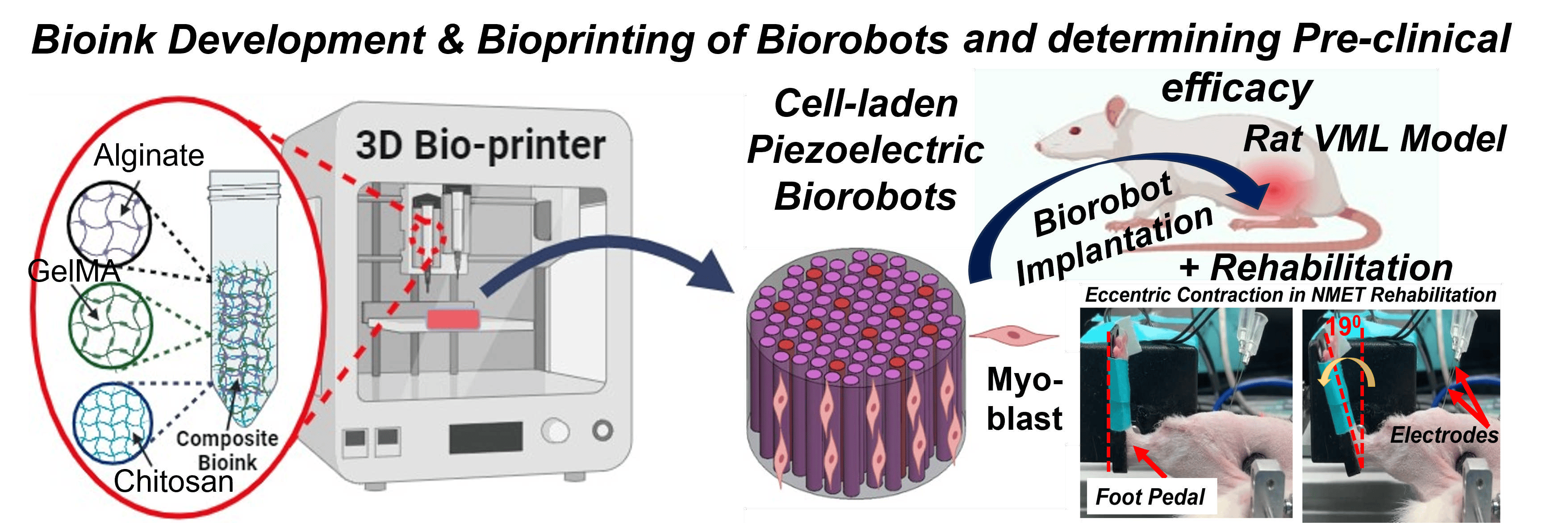
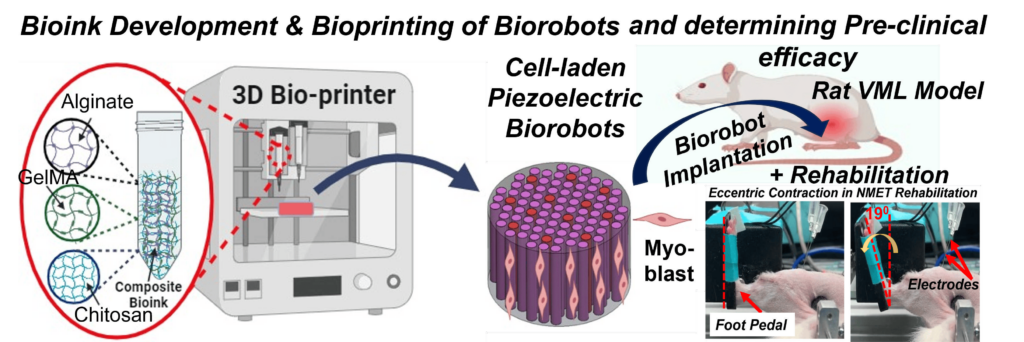
1. An Intelligent Bioprinted Biorobot for Regenerative Rehabilitation of Volumetric Muscle Loss
Volumetric Muscle Loss (VML) is one of the most severe and chronic musculoskeletal injuries, often resulting from traumatic incidents and characterized by the loss of a significant portion of skeletal muscle. One of the major barriers in existing regenerative approaches is the inability to effectively reinnervate newly formed muscle tissue—an essential requirement for restoring functional movement.
This project pioneers a next-generation Regenerative Rehabilitation (Regen-Rehab) strategy to address this critical gap. Our goal is to develop and evaluate a pre-clinical intelligent bioprinted biorobot—a piezoelectric muscle construct that not only delivers cells but also generates low-level electrical stimulation (E-Stim) autonomously using mechanical forces from natural limb movements. This smart stimulation accelerates muscle regeneration, reinnervation, and vascularization.
Using advanced cell-laden bioprinting, we are engineering this biorobot to mimic the native muscle environment. To further boost therapeutic outcomes, we are integrating it with a physical rehabilitation protocol known as neuromuscular eccentric contraction training (NMET)—bridging engineered regeneration with active rehabilitation.
This multidisciplinary project leverages innovative in vitro and in vivo models to evaluate the functionality, integration, and regenerative potential of the biorobot in treating VML.
Funded by a $3 million, 5-year NIH R01 grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), this work sets the stage for intelligent, responsive, and clinically translatable therapies for complex muscle injuries.
Learn more about the project here: https://reporter.nih.gov/search/RVhyy0-cFke8eTPb_3L78g/project-details/11043168
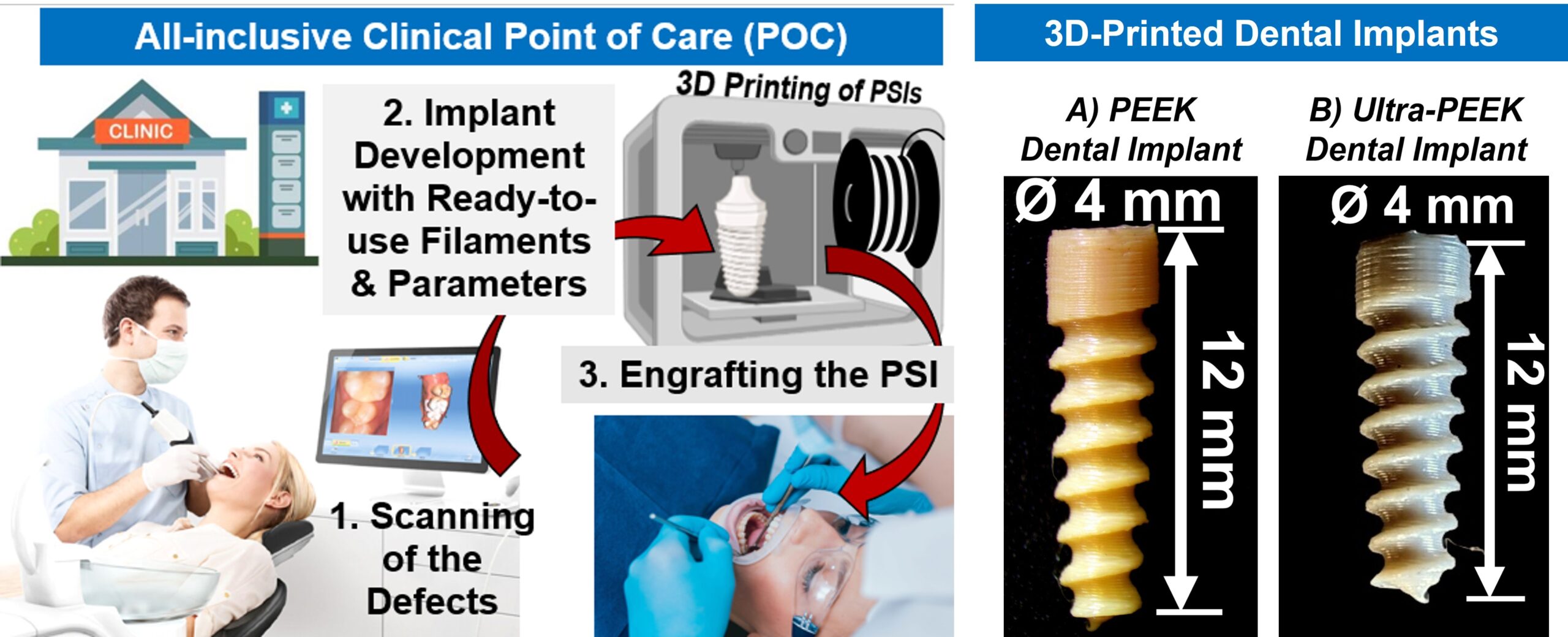
2. Ultra-PEEK: 3D-Printed Dental Implants to Combat Peri-Implantitis
Peri-implantitis is one of the most prevalent and difficult-to-treat oral infections, affecting millions of patients with dental implants each year. Our lab is leading an innovative project to engineer a next-generation, patient-specific dental implant that not only resists bacterial infection but also promotes strong integration with the surrounding bone.
At the heart of this project is Ultra-PEEK—a multifunctional composite made by incorporating silver-doped amorphous magnesium phosphate (AgAMP) bioceramic particles into a high-performance biopolymer known as polyetheretherketone (PEEK). This unique combination provides both antibacterial and bioactive properties, enhancing the implant’s ability to prevent infection and support osseointegration.
What sets Ultra-PEEK apart is its compatibility with Fused Filament Fabrication (FFF)-based 3D printing, allowing us to fabricate custom-designed implants with high precision and mechanical durability that meet FDA requirements.
This highly interdisciplinary project brings together experts in mechanical engineering, materials science, additive manufacturing, and dentistry—including oral and maxillofacial surgeons and endodontists.
Funded by a $3.3 million, 5-year NIH R01 grant from the National Institute of Dental and Craniofacial Research (NIDCR), this work represents a major step forward in personalized, infection-resistant dental implant technology.

Learn more about the project here: https://reporter.nih.gov/search/RVhyy0-cFke8eTPb_3L78g/project-details/10980069
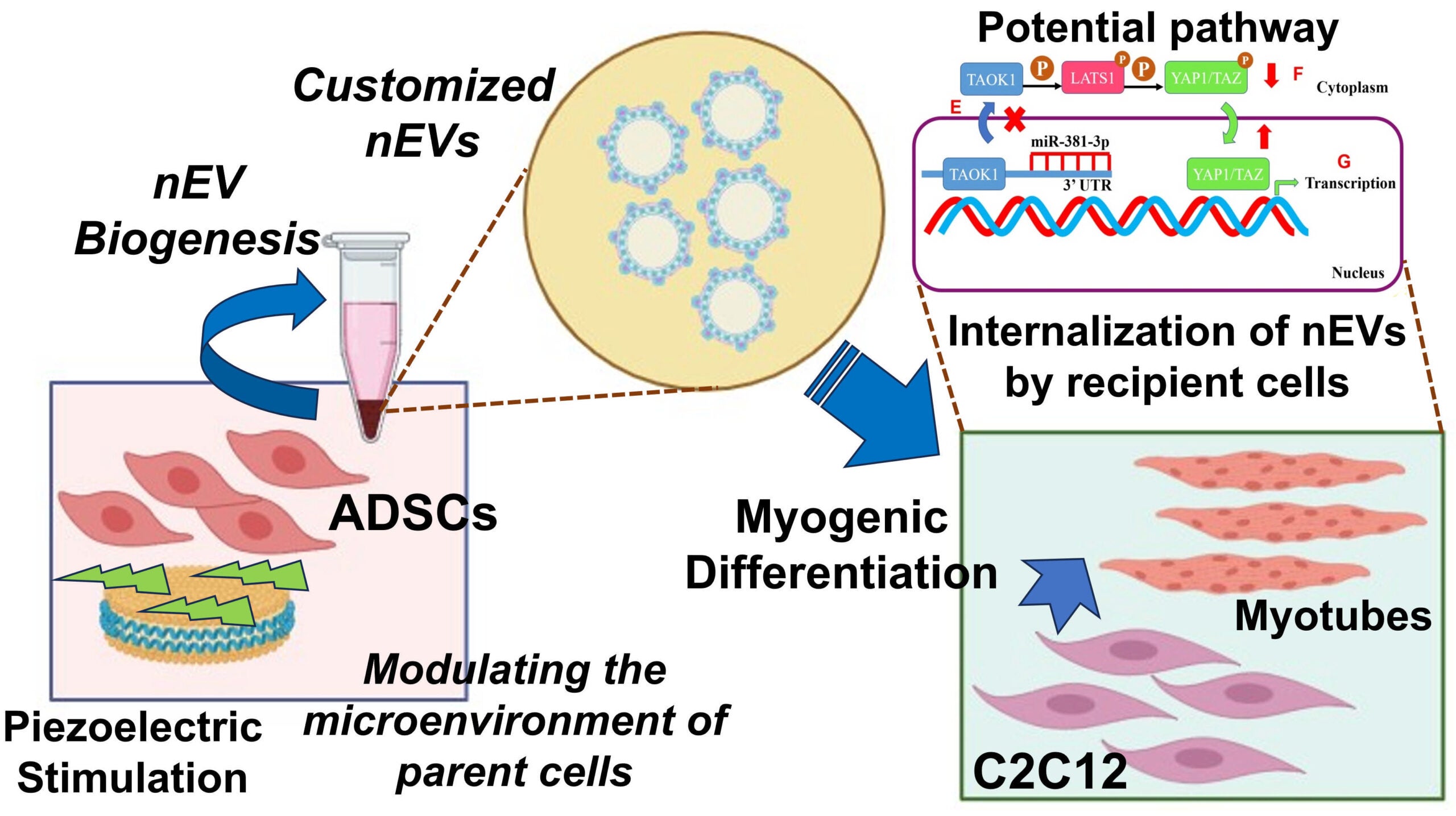
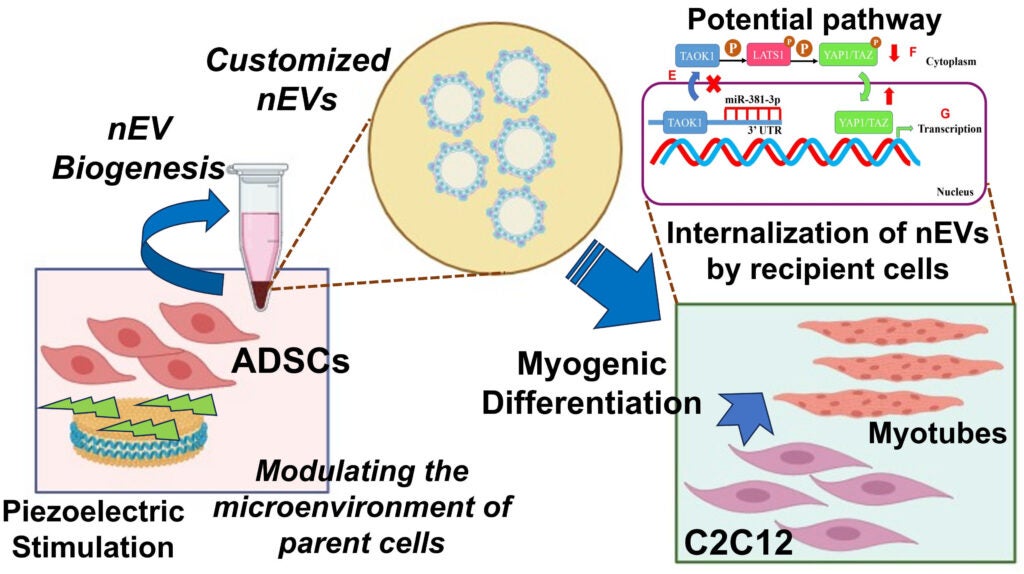
3. Engineering Customized Exosomes for ‘Cell-Free’ Therapy of Volumetric Muscle Loss (VML)
Volumetric Muscle Loss (VML) is a devastating musculoskeletal injury involving the irreversible loss of large portions of muscle due to trauma or surgery. It often leads to lifelong disability and, in severe cases, can be life-threatening. Current clinical treatments—such as surgical grafting or implantation of synthetic scaffolds followed by rehabilitation—offer limited success and often fail to restore functional muscle.
While cell-based tissue engineering holds promise, it also presents challenges such as immune rejection and inflammation. As an alternative, extracellular vesicles (EVs)—naturally secreted, cell-derived particles—have emerged as powerful ‘cell-free’ regenerative therapies. These nanoscale vesicles can promote tissue repair without the risks associated with live-cell transplantation.
Our project aims to bioengineer customized therapeutic exosomes (a subtype of EVs, known as nEVs) to drive muscle regeneration in VML injuries. By applying precisely controlled piezoelectric stimulation to adipose-derived mesenchymal stem cells (ADSCs), we program their exosomal output to enhance myogenesis—the formation of new muscle tissue—in recipient muscle cells.
This pilot study lays the groundwork for a novel, immune-safe, and highly targeted regenerative approach to treating VML, potentially transforming the way we manage large-scale muscle injuries.
This research is supported by an NIH R03 grant from the National Institute of Biomedical Imaging and Bioengineering (NIBIB).
Learn more about the project here: https://reporter.nih.gov/search/RVhyy0-cFke8eTPb_3L78g/project-details/10937296